The company intends to increase sales in the UK to approximately 2 million Euros by focusing on seven molecules, which are already registered in the country.
Venus Pharma GmbH, the company's German subsidiary, has secured marketing authorisation from the UK for Cisplatin, a chemotherapy drug used as a first-line treatment for advanced ovarian cancer, testicular cancer and bladder carcinoma. This will enable the company to offer its affordable range of cancer drugs to the National Health Service in the UK and neighbouring countries.
The company intends to increase sales in the UK to approximately 2 million Euros by focusing on seven molecules, which are already registered in the country.
The registration for Cisplatin will also expedite the process of registering the oncology products of Venus Remedies in many other countries around the world, which consider the UK as a reference country for fast-tracking registration and open opportunities for supplying unregistered products to several markets globally.
The global market size of Cisplatin was valued at $394.5 million in 2021 and is expected to grow to $661.16 million by 2027 at a compounded annual growth rate (CAGR) of 8.99 percent. The UK holds at least 5 percent of the global market share for this drug.
“We plan to achieve 2 million euros in sales in the UK focussing on seven molecules, which are already registered in the UK and augmenting their sales by 20 percent on a year-on-year basis. We are also planning to add new molecules in the UK and other European countries, which will help us expand our operations,” Saransh Chaudhary, President Global Critical Care, Venus Remedies said.
Venus Remedies has received marketing authorisations from the UK Medicines and Healthcare products Regulatory Agency (MHRA) for drugs including Gemcitabine, Bortezomib, Meropenem, Docetaxel, Carboplatin and Ceftazidime
Obtaining marketing authorisations for drugs in the UK will help the company strengthen its position in regulated markets in the Middle East.
Registration for Cisplatin in the UK could potentially help Venus Remedies in expediting the registration of its oncology product Paclitaxel, which is pending with the MHRA.
“We are also about to apply for the marketing authorization for Bendamustin a chemotherapy medication used in the treatment of chronic lymphocytic leukaemia, in the UK,” Saransh Chaudhary added.
Also Read | Vaccine against cervical cancer to be included in national immunisation programme by mid-2023: NTAGI chief
In February, Venus Remedies got the Saudi GMP nod for all its production facilities in Baddi and marketing approval for cancer drugs from Uzbekistan, and Palestine.
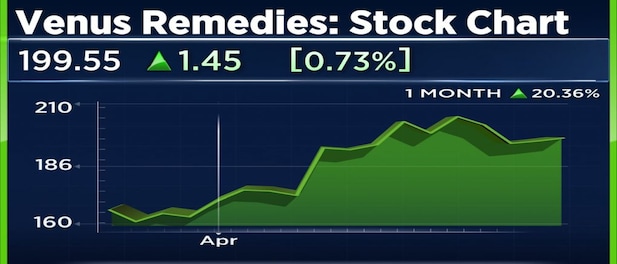
Shares of Venus Remedies are trading 2 percent higher and are near day’s high of Rs 208 following the UK MHRA nod. Shares have risen more than 11 percent year-to-date.
Check out our in-depth Market Coverage, Business News & get real-time Stock Market Updates on CNBC-TV18. Also, Watch our channels CNBC-TV18, CNBC Awaaz and CNBC Bajar Live on-the-go!